TOC System Suitability Testing
The United States Pharmacopeia (USP) Chapter <643> outlines the general method for Total Organic Carbon (TOC) testing in pharmaceutical applications. This chapter provides guidance on how to qualify the analytical technique for use, as well as guidance on how to interpret TOC instrument results for use as a limit test.
USP Chapter <643> and European Pharmacopeia (EP) Chapter 2.2.44 are closely harmonised. The suitability of the TOC analyser is determined by testing a certified blank (Reagent Water, Rw) a standard solution of 0.500mg C/L sucrose (Rs) dissolved in Reagent Water, and a System Suitability Solution of 0.500mg C/L of 1,4-benzoquinone (Rss) also dissolved in Reagent Water. Rs is intended as a relatively “easy to oxidise” standard & Rss as a “difficult to oxidise” standard.
After analysing all 3 samples, it is possible to calculate the Limit Response (Rl) & Response Efficiency (Re). The test solution must measure below the calculated limit response in order to meet the requirements of Chapter <643> and Chapter 2.2.44. The Response Efficiency is a representation of the instrument’s ability to oxidise different types of organic compounds. The system is determined to be suitable if the Response Efficiency lies between 85% and 115%.
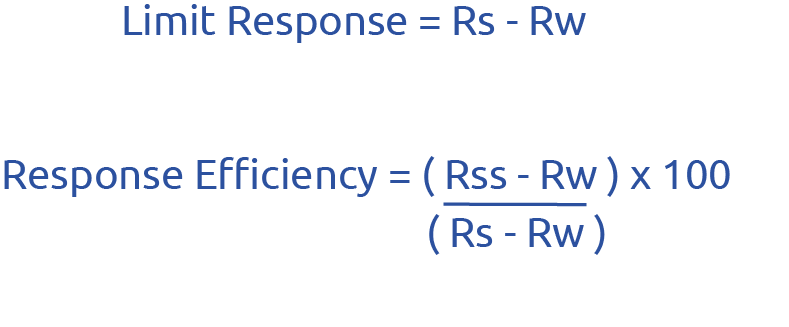

With the release of USP 37, the TOC Chapter <643> was revised to include Sterile Water for Injection (SWFI), Sterile Purified Water (SPW), Sterile Water for Irrigation and Sterile Water for Inhalation. For Sterile Water quality, the testing procedure was slightly modified, and higher limits were established.
USP Chapter <643> suggests TOC System Suitability Testing (SST) should be performed ‘periodically’ while the European Pharmacopeia Chapter <2.2.44> prescribes ‘suitable’ intervals for TOC System Suitability testing.
Therefore, the frequency needs to be established considering the risk associated with the system being out of tolerance, and the cost to demonstrate that the system is within tolerance. The frequency of TOC System Suitability testing is a quality control decision. Test data used to establish SST frequency should be documented and used as evidence for the most appropriate frequency.

For new water plants or TOC analysers, it is recommended to perform SST on a more frequent basis as part of the system’s performance qualification. Frequency may be reduced once sufficient data is available to demonstrate performance of the instrument.
For laboratory analysers, customers typically choose to run SST daily or weekly for a set period. With the use of statistical process control, the performance of SST may be extended to longer intervals if the analytical data demonstrates stability over the longer period.
Use of externally supplied Certified Reference Materials (CRMs) for SST purposes is preferred as this offers the most rigorous assessment of an instrument’s performance. CRMs from an independent ISO17025 & ISO17034 Accredited Certified Reference Material producer ensures full traceability & process accountability.
For other news posts, please visit our News Page.

