What is Total Organic Carbon? Why is its measurement important in pharmaceutical applications?
What is Total Organic Carbon (TOC)?
Total Organic Carbon is the amount of carbon bound in an organic compound. It is ubiquitous and exists in many forms. It is a reliable, non specific indicator of water quality.
Why should we measure Total Organic Carbon (TOC)?
TOC analysis is a quantitative measure used to establish all of the organic carbon in a compound, regardless of its structure. Compared to other more specific forms of analysis, TOC is an easy-to-measure and rapid way of gaining an indicator of the level of organic contamination.
USP method <643> on Total Organic Carbon says:
“TOC is an indirect measure of organic molecules present in pharmaceutical waters measured as carbon. Organic molecules are introduced into the water from the source water, from purification and distribution system materials. TOC can also be used as a process control attribute to monitor the performance of unit operations comprising the purification and distribution system.”
TOC is used extensively to control the cleanliness of pharmaceutical waters such as Purified Water (PW), Ultra Purified Water (UPW), Water for Injection (WFI) and Sterile Water. These waters are used throughout the pharmaceutical manufacturing process for cleaning. Some of them also make up a substantial part of the final drug being manufactured.
Contaminants in Pure Waters
Measuring and maintaining Total Organic Carbon (TOC) levels is crucial for ensuring that no harmful toxins are released into the Water Systems from source water, the purification system and/or points of use. In the pharmaceutical industry, high purity water is one of the main components used throughout a number of production processes, and therefore, it is essential to ensure that the water is of a certain purity to prevent contamination or poor product quality.
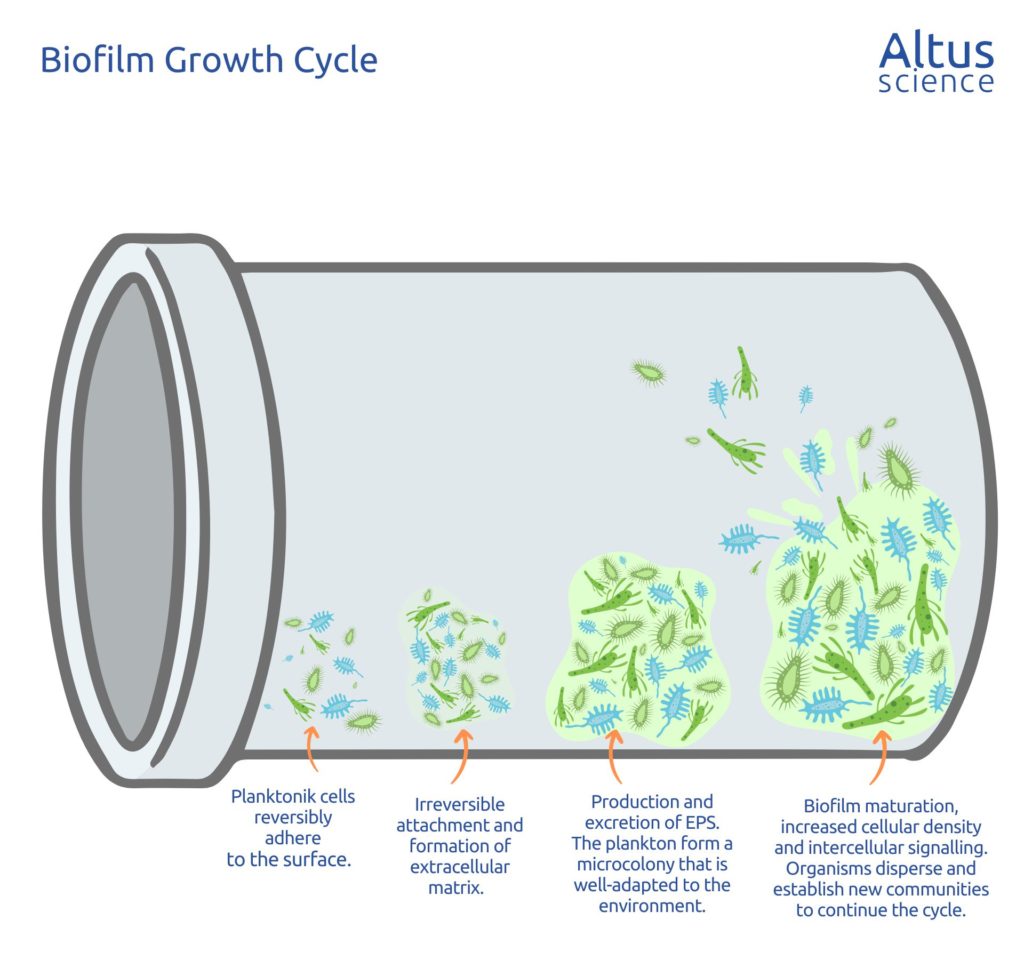
TOC can be a good indicator of biofilm build-up. A water system undergoing a slow accumulation of biofilm may encounter Total Organic Carbon (TOC) spikes within the distribution network following sanitation cycles, as the biofilm detaches from the inner walls of the pipes. TOC is also useful in testing the cleaning methods used by companies. By ensuring that their water supply cleaning systems are running correctly, organisations can minimise the risk of low-quality water being used in the manufacturing process.
The Importance of measuring TOC in pharmaceutical manufacturing
When administering drugs, either orally or via injection, we naturally want to minimise the amount of contaminants present. When we drink water, the water molecules can move directly through the phospholipids of the plasma membrane, this is called osmosis.
When we inject drugs such as vaccines, we bypass our bodies natural osmosis system. Water For Injection (WFI) is commonly used as a solvent/carrier for liquid injections. WFI is typically >95% of the bulk of the injection, excipients, parenteral and active parenteral ingredients make up <5%. Injecting directly bypasses the bodies osmosis system and it is therefore critical that the organic contaminants in WFI are tightly controlled.
Purified Water (PW) is often used to make up oral dosing. Oral dosing PW is typically >90% of the liquid, excipients, parenteral and active parenteral Ingredients make up <10% It is critical that TOC is controlled in PW as otherwise there would be potential for an organic compound to react with other components in oral dose formulation, forming something potentially toxic to patients.
Purified Water (PW) & Ultra Purified Water (UPW) are also used for cleaning in pharmaceutical processes. Again, it’s critical that TOC levels are controlled to avoid the possibility of batch contamination.
Each of the global pharmacopeias release compendia annually and most have a chapter dedicated to TOC. There is close harmonisation between leading pharmacopeias. The USP (United States Pharmacopeia) chapter for TOC is USP 643, the EP (European Pharmacopeia) chapter is 2.2.44. JP (Japanese Pharmacopoeia) also states requirements for TOC compliance.
All chapters state a nominal limit for TOC of 500ppb or 0.500mg/L C. It is therefore a compendia requirement that Pharmaceutical manufacturers wishing to comply with any of these chapters measures and controls TOC. The ultimate goal of these requirements is to improve patient safety.
As well as for pure water analysis, TOC analysis is commonly used for Cleaning Validation. The speed of TOC analysis makes it a good option compared to more specific methods such as HPLC.
Want to find out more about TOC and its measurement? Check out our other articles here.
Altus Science manufacture and supply ISO 17025 & ISO 17034 Accredited TOC Standards to the pharmaceutical industry. Contact us today to see how we can support you.

